The Rare Earth Elements
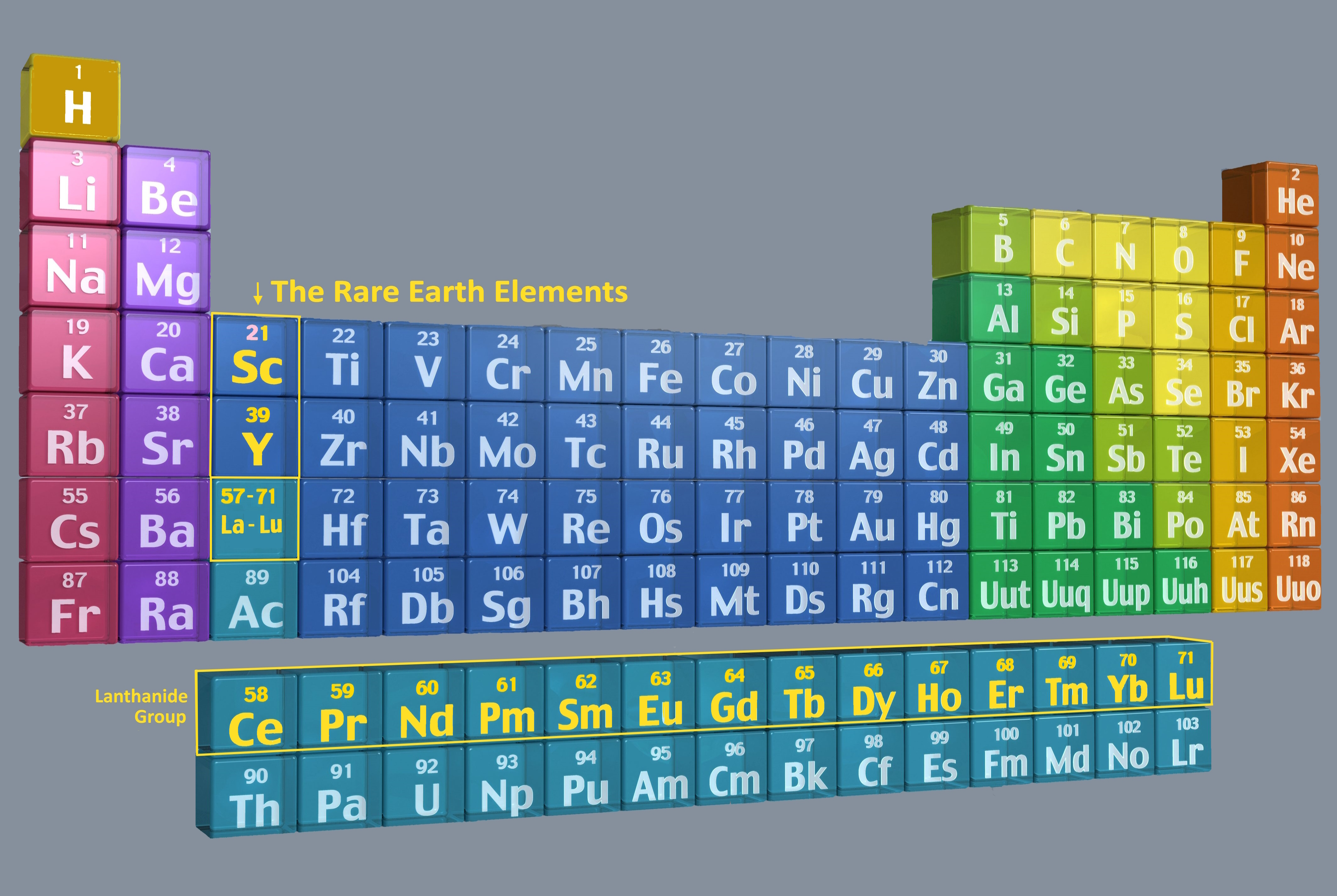
| As electronics have become ubiquitous in our lives, for everything from smartphones to flat-screen TV's to electric vehicles, we hear more an more stories in the news about Rare Earth elements (REE's). Just a few years ago, most people would not have even recognized the term. Now we are beginning to understand that they have become critical raw materials. The Rare Earths are actually 17 distinct metallic elements. They include the entire Lanthanide Group of the periodic table (atomic numbers 57-71), plus scandium (21) and yttrium (39) being the others. Most are actually fairly common in the earth's crust, but economically-minable concentrations are not widespread and they are challenging to process into the pure metals. Deposits typically contain multiple members of the group in varying proportions. Members of the group have differing properties that account for their industrial value. These include powerful magnetism, electroluminesce, and chemical catalytic behavoior. They are often classified by their atomic wights as either light or heavy REE's, besed on some common variations in properites. It is common to hear metals like Lithium and Cobalt included in discussions of the REE's, simply because they are also important to the electric vehicle industry, but that is incorrect and they are completely different chemically. Decades ago, when demand was much lower, the Unitied States was the largest producer. However, the discovery of several very large deposits in China has led to that country now accounting for some 80% of world production. 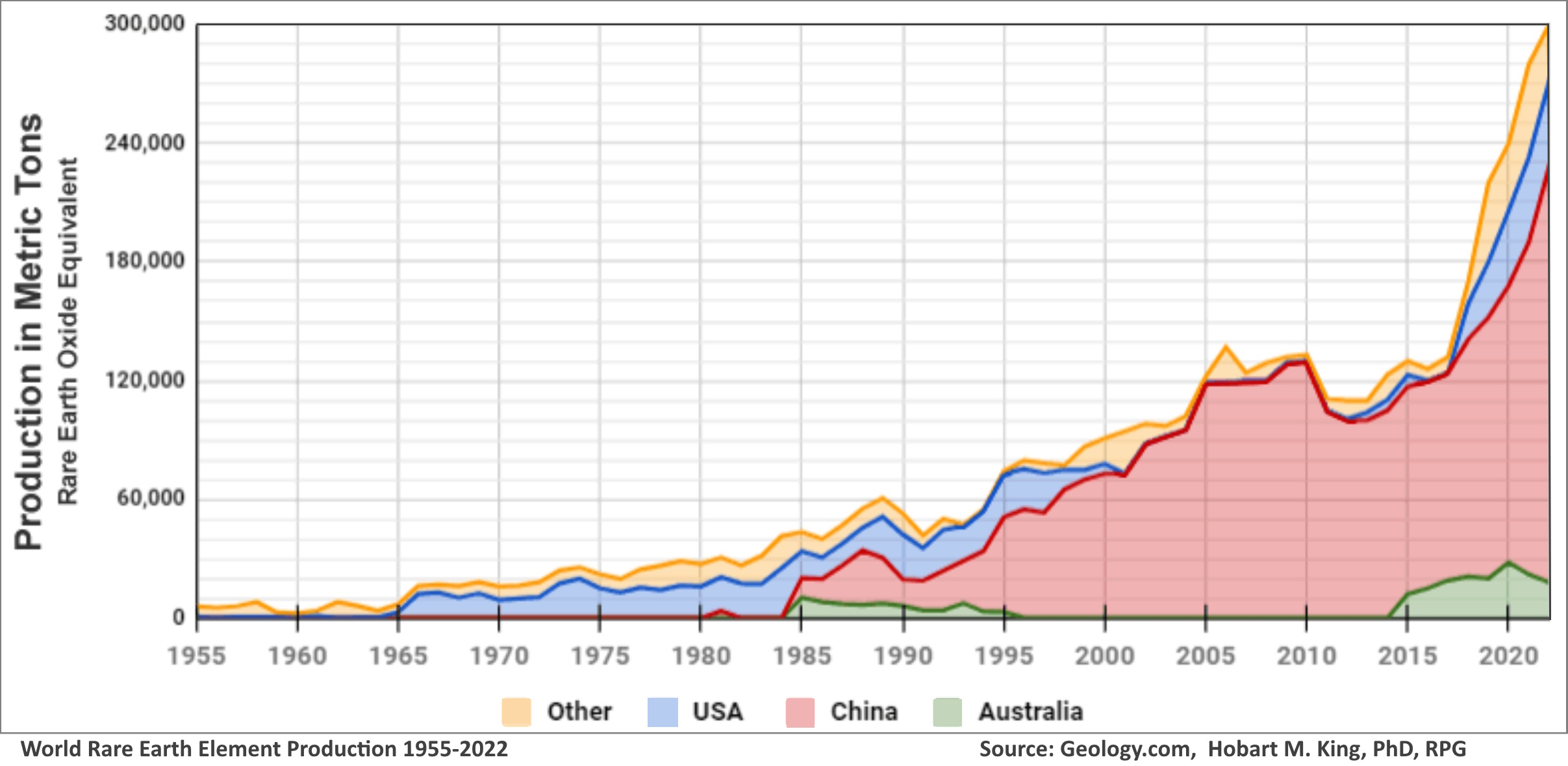
Below is a full list of the 17 REE's. The pictures are sourced from the Time-Life publication, Matter © 1963. Note that some are shown in glass containers filled with inert argon gas, as they are particularly reactive with oxygen. Each element name is hyperlinked to an excellent article by the Royal Society of Chemistry with extensive information including chemical and physical properties, uses and supply risks, and history. There are even videos. One interesting fact is that four of the elements are named for the single town of Ytterby, Sweden where they were first discovered. |
|
| Scandium |
| Atomic number 21. Generally grouped with the light REE's. A First Transition Metal in the periodic table, but grouped with the REE's due to its similar chemical properties. Uses include alloys, specialized lighting, and oil exploration. | |
|
| Yttrium |
| Atomic number 39. Generally grouped with the heavy REE's. A First Transition Metal in the periodic table, but grouped with the REE's' due to its similar chemical properties. Most prevalent uses are for alloys and microwave filters for radar. | |
|
| Lanthanum |
| Atomic number 57 (light REE). Lanthanum lends its name to the Lanthanide Group, which includes all of the subsequent elements in this list. Uses include alloys, lighting, and optics. | |
|
| Cerium |
| Atomic number 58 (light REE). Common uses include alloys, catalysis, and lighting. | |
|
| Praseodymium |
| Atomic number 59 (light REE). Uses include alloys - especially for magnets, and coloring for glass. | |
|
| Neodymium |
| Atomic number 60 (light REE). It is very important as the major component in alloys for powerful magnets, as well as specialized optics and lasers. | |
|
| Promethium |
| Atomic number 61 (light REE). Promethium is radioactive and finds some usage as a radiation source, but is not considered a critical REE. | |
|
| Samarium |
| Atomic number 62 (light REE). Use in magnetic alloys for high-temperaure use, as well as a neutron absorber in nuclear energy applications. | |
|
| Europium |
| Atomic number 63 (light REE). Used in specialize optics, lasers, and also as a neutron absorber in nuclear energy applications. In the early days of color television it was an imporant phosphor. | |
|
| Gadolinium |
| Atomic number 64 (light REE). Gadolinium-based "dyes" are used in MRI diagnostics. It is also an excellent neutron absorber in nuclear energy applications. | |
|
| Terbium |
| Atomic number 65 (heavy REE). Used in electronic components, x-ray imaging, and accoustics. | |
|
| Dysprosium |
| Atomic number 66 (heavy REE). Used as an alloying compoenent to increase the high-temperature tolerance of Neodymium-based magnet, as well as for nuclear applications as a neutron absorber. | |
|
| Holmium |
| Atomic number 67 (heavy REE). Its primary use is as a neutron absorber in nuclear applications. | |
|
| Erbium |
| Atomic number 68 (heavy REE). Used primarily in alloys and specialized optics. | |
|
| Thulium |
| Atomic number 69 (heavy REE). Used for small-scale X-ray emitters and for lasers. | |
|
| Ytterbium |
| Atomic number 70 (heavy REE). It is finding increasing use in electronics, lasers, and as a catalyst. | |
|
| Lutetium |
| Atomic number 71 (heavy REE). Not heavily used, but has some application as a catalyst in oil refining. |

 The only large producing deposit in the United States is the Mountain Pass Mine in the Southern California desert near the Nevada Border, about 53 miles from Las Vegas. It was discovered in 1949 and increased production rapidly from the mid 1960's as electronics demand grew, particularly color television sets which used europium. It also supplied significant quantities of cerium, lanthanum, neodymium and praseodymium. The latter two elements are particularly important today for high-power magnets used in electric car motors and a wide variety of consumer and defense products. After Chinese production increased dramatically in the late 1990's, economic and environmental challenges resulted in the mine's shutdown, but with the subsequent increase in market demand and price it has again reached high production under the new managment, MP Materials Corp.
The only large producing deposit in the United States is the Mountain Pass Mine in the Southern California desert near the Nevada Border, about 53 miles from Las Vegas. It was discovered in 1949 and increased production rapidly from the mid 1960's as electronics demand grew, particularly color television sets which used europium. It also supplied significant quantities of cerium, lanthanum, neodymium and praseodymium. The latter two elements are particularly important today for high-power magnets used in electric car motors and a wide variety of consumer and defense products. After Chinese production increased dramatically in the late 1990's, economic and environmental challenges resulted in the mine's shutdown, but with the subsequent increase in market demand and price it has again reached high production under the new managment, MP Materials Corp.  A massive deposit is sea-floor sediments exists
A massive deposit is sea-floor sediments exists 

















